The pharmaceutical industry is on the brink of a technological revolution, with cloud computing at its core. Projected to grow to USD 59.0 billion by 2032, this market is expected to expand at a compound annual growth rate (CAGR) of 14.6% from 2024 to 2032.
Cloud computing helps pharmaceutical companies in the following ways:
- It uses online tools and storage to improve their work and collaboration.
- It organizes large amounts of data from drug development, like clinical trial results and patient records, into one easy-to-access place.
- This technology breaks down barriers between data sources, improving information sharing and supporting teamwork across research projects.
This blog will explore how cloud computing is transforming the pharmaceutical industry and why it is becoming essential and making the drug development process more connected and efficient.
Challenges in Cloud Adoption for the Pharmaceutical Industry
Due to several challenges, the pharmaceutical sector has been cautious in adopting cloud technologies. Known for its strict regulations and critical operations, the industry faces unique obstacles that complicate the shift to digital solutions. In this discussion, we’ll look at the main reasons for this hesitation, including regulatory issues, the effectiveness of cloud solutions, and the financial costs involved in moving to the cloud.
Regulatory Challenges
Pharmaceutical companies are bound by strict regulations that demand comprehensive validation and verification of their systems. This requirement ensures that technological solutions, including cloud systems, are reliable and compliant with industry standards. However, validating these systems can be costly and time-consuming, often consuming between 20% and 30% of the allocated budget for software implementation in cloud-hosted solutions.
Solution Efficacy and Integration Costs
Adopting new technologies involves significant expenditure not only on the software itself but also on integrating it into existing business systems. Costs can escalate due to needs such as extended employee training, new hires, and necessary upgrades to existing infrastructure. Moreover, there’s a perceived risk associated with new systems, which requires extensive testing and operational controls to ensure their effectiveness and compliance.
Data Security and Sovereignty
Moving sensitive data from in-house servers to the cloud introduces potential security risks. The responsibility for ensuring data security shifts to the cloud provider, raising concerns, especially if trust issues exist between the pharmaceutical company and the provider. Data sovereignty issues arise when data is stored in countries different from where the company operates, complicating compliance with local legal regulations.
Despite these challenges, there are successful examples of pharmaceutical companies benefiting from cloud solutions, such as UCB’s collaboration with Microsoft and Pfizer’s partnership with AWS, which have enhanced their drug development processes. These instances demonstrate that the cloud can significantly benefit the pharmaceutical industry with the right approach and solutions.
Advantages of Cloud Computing in the Pharmaceutical Industry
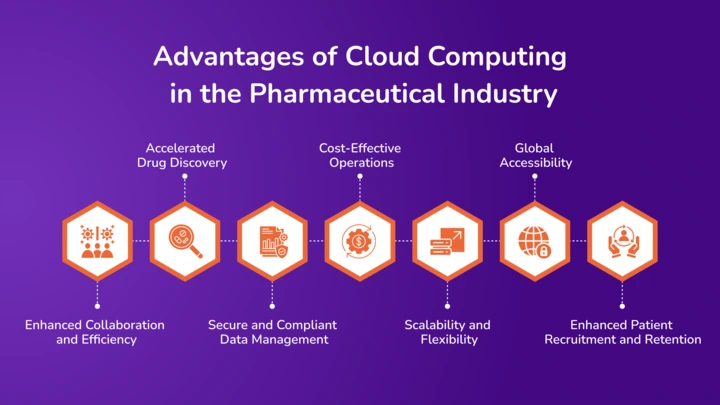
Cloud computing is transforming the pharmaceutical industry by providing powerful tools for innovation and efficiency. Below, we explore the advantages of cloud computing in the pharmaceutical industry:
Enhanced Collaboration and Efficiency
Cloud technology enables seamless collaboration across geographical boundaries, essential for modern pharmaceutical operations. Here’s how it helps:
- Virtual Workspaces: Professionals can share findings and collaborate in real time, enhancing research and development speed.
- Production Line Management (PLM): Cloud-based PLM systems allow for continuous alignment between various stakeholders throughout the drug development lifecycle, ensuring efficient resource utilization and reduced time to market.
Accelerated Drug Discovery
Cloud computing offers computing power and storage capabilities, significantly speeding up drug discovery. By utilizing data analytics and advanced algorithms, cloud platforms can process and analyze extensive datasets much faster than traditional methods, including molecular structures and clinical data. Studies show that cloud computing can reduce the time for drug discovery by up to 50%, thanks to efficient data handling and processing capabilities.
Secure and Compliant Data Management
A report indicates that cloud platforms reduce the risk of data breaches by up to 40% compared to traditional IT environments. The cloud offers strong security features that protect sensitive clinical trial data and ensure compliance with stringent regulatory standards.
- Data Encryption: Ensures data is secure at rest and in transit.
- Access Controls: Only authorized personnel can access sensitive information, enhancing data integrity and security.
- Compliance: Cloud providers often adhere to international security standards (e.g., HIPAA, GDPR), simplifying compliance for pharmaceutical companies.
- Automatic Backups: Cloud systems automatically back up data regularly, safeguarding against data loss due to hardware failure or cyber-attacks.
- Centralized Storage: Data from various sources and locations is centralized in a secure cloud environment, making it easier to manage, access, and analyze.
Cost-Effective Operations
Cloud computing reduces the need for heavy upfront investments in IT infrastructure and ongoing maintenance costs. Pharmaceutical companies report, on average, a 30% reduction in IT operational costs after adopting cloud services. Through a pay-as-you-go model, pharmaceutical companies can scale resources up or down based on current needs, which is especially beneficial for managing the fluctuating demands of clinical trials and drug development projects.
Scalability and Flexibility
Cloud computing allows for quick adjustment of computing resources to meet the changing demands of the pharmaceutical industry. Companies can easily scale computing power during peak periods, such as large-scale clinical trials, and scale down during slower periods to optimize resource usage and minimize costs.
Global Accessibility
Cloud technology enables global access to data and tools, which is crucial for pharmaceutical companies with operations and partnerships across multiple continents. According to industry reports, cloud adoption has led to a 50% improvement in project timelines for multinational pharmaceutical trials. With cloud services, team members in different regions can access the same resources and data in real time, facilitating quicker decision-making and ensuring consistency across global operations.
Statistics:
Enhanced Patient Recruitment and Retention
Cloud computing significantly improves patient recruitment and retention in clinical trials by enabling more targeted and efficient recruitment strategies. This advantage is particularly crucial in ensuring the success and validity of clinical trials.
- Data Analytics and AI: Cloud-based platforms can utilize data analytics and artificial intelligence to analyze demographic and medical data, helping to identify the most suitable candidates for specific trials.
- Remote Engagement: Cloud services facilitate remote and decentralized trials, reducing the need for frequent site visits and making it easier for patients to participate, which can help reduce dropout rates.
Utilizing cloud technology in clinical trials has been shown to increase patient enrollment rates by up to 20% and reduce dropout rates by as much as 15%, enhancing the overall efficiency and effectiveness of the trials. With cloud computing, pharmaceutical companies can reach a wider and more appropriate audience, improve enrollment rates, and enhance patient adherence to trial protocols, leading to more robust and reliable trial outcomes.
Use Cases of Cloud Computing in the Pharmaceutical Industry
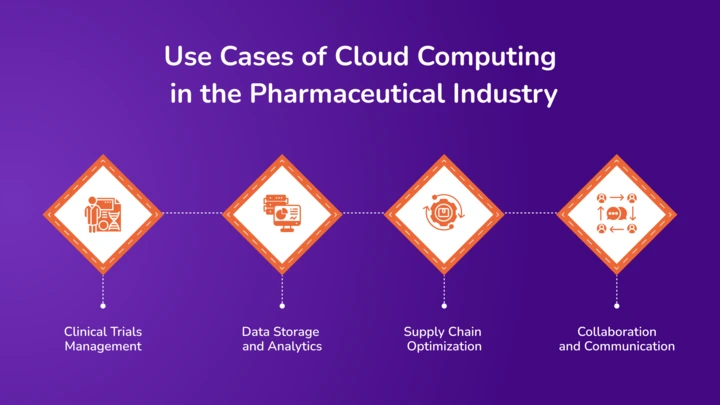
Cloud computing enables companies to manage vast data sets and complex processes more effectively, paving the way for drug development and patient care advancements.
- Clinical Trials Management
Cloud-based platforms are increasingly used in pharmaceutical clinical trials to manage and analyze vast data. These platforms enable real-time access to trial data, allowing for patient monitoring and data collection remotely, making the process more efficient. This enhances patient recruitment and retention, improves data accuracy, and reduces the time and cost associated with clinical trials.
- Data Storage and Analytics
Cloud computing provides scalable storage solutions and advanced analytics capabilities to handle vast amounts of data generated in pharmaceutical research. This enables efficient data management, supports regulatory compliance, and fosters insights through big data analytics, leading to better decision-making.
- Supply Chain Optimization
Cloud-based solutions integrate various supply chain elements, from manufacturing to distribution, ensuring efficient resource management and real-time monitoring, improving supply chain visibility, reducing operational costs, and enabling quick adaptation to market changes or disruptions.
- Collaboration and Communication
Cloud-based computing facilitates seamless collaboration across pharmaceutical companies’ different geographical locations and departments. It supports virtual teams, enhances communication between researchers, and accelerates the innovation process by efficiently sharing data and tools.
These use cases highlight how cloud computing is transforming the pharmaceutical industry, driving efficiency, innovation, and compliance across various facets of the sector.
Real-World Examples of Cloud Computing in the Pharmaceutical Industry
Cloud computing revolutionizes the pharmaceutical industry, providing dynamic solutions that enhance efficiency and foster innovation. Here are some of the examples:
Challenge
Pfizer dealt with vast amounts of data from drug discovery and clinical trials, which overwhelmed their traditional IT infrastructure. Rapid scaling up for high-performance computing tasks, like genomic analysis, was cumbersome and slow.
Solutions with Cloud Computing
Using AWS, Pfizer could leverage scalable computing resources to process and analyze large datasets efficiently. Using cloud services, Pfizer reduced the overhead costs of maintaining large-scale data centers.
Challenge
Novartis needed advanced computational capabilities to simulate drug interactions and formulations, which was not feasible with their existing computing resources. Accelerating the pace of innovation was crucial to staying competitive, but existing technological limitations hindered it.
Solutions with Cloud Computing
By partnering with Microsoft Azure, Novartis leveraged AI to model and predict drug interactions, speeding up the formulation process. Cloud platforms enabled seamless collaboration between researchers across the globe, facilitating faster innovation cycles.
Challenge
AstraZeneca needed to efficiently process vast amounts of genomics sequencing data to support its drug discovery and genomics research. The scale of the data was immense, requiring robust computational power to handle billions of statistical tests swiftly. The urgency to accelerate the genomics data analysis process was crucial for timely insights that could influence numerous drug discovery projects.
Solutions with Cloud Computing
Utilizing AWS, AstraZeneca implemented a cloud-based solution incorporating AWS Lambda for serverless computing, AWS Batch for efficient batch computing, and Amazon S3 for scalable storage. This setup enabled the rapid and scalable processing of genomics data. The integration of AWS services automated the data orchestration and task execution, significantly reducing the time needed for genomic analyses and allowing the team to perform over 51 billion statistical tests in less than 24 hours.
Challenge
Fringe needed to scale its AWS infrastructure to handle growing customer demands and maintain security compliance but needed more in-house capacity to manage these changes effectively. As a startup, Fringe was also looking for a cost-effective solution to enhance its infrastructure without significantly expanding its DevOps team.
Solutions with Cloud Computing
Avahi redesigned Fringe’s AWS environment to utilize Infrastructure as Code (IaC) and serverless computing, enabling automatic scaling and efficient resource management. Avahi implemented Amazon Elastic Kubernetes Service (EKS) to manage containerized applications’ deployment, scaling, and operations. They also integrated various AWS security tools such as Amazon GuardDuty and Amazon Inspector to enhance threat detection and ensure SOC2 compliance.
By utilizing cloud computing, the pharmaceutical industry enhances its operational efficiencies and positions itself at the forefront of technological innovation, driving faster, safer, and more cost-effective drug development and distribution.
Elevate Your Business with Avahi’s Cloud Expertise!

Avahi specializes in delivering innovative cloud solutions that address your unique business challenges. As your dedicated AWS Cloud Consulting Partner, we are committed to simplifying your cloud journey through seamless adoption, migration, and application modernization.
Our Services Include:
- Adoption and Migration: Migrate your legacy applications or workloads to AWS for enhanced reliability, scalability, and performance.
- DevOps: Unlock the power of your data with advanced analytics and machine learning capabilities.
- Cloud Staffing Services: Access top-tier talent to support and drive your cloud initiatives, ensuring a seamless and effective cloud transformation.
Want to explore how Avahi can help you simplify, modernize, and excel in your cloud journey.